Refractory Chronic Cough (RCC) Therapy May Be One of the Most Asymmetric Bets in Respiratory Medicine
For decades, refractory chronic cough (RCC) has occupied an uncomfortable space in respiratory medicine: highly prevalent, clinically burdensome, and commercially invisible.
That era is ending.
A newly released Market Report: Refractory Chronic Cough (RCC) Therapeutics: Pipeline Analysis & Competitive Landscape 2025–2035 provides the most complete assessment to date of why - and how - this category is finally becoming investable.
The headline paradox is striking. An estimated ~100 million people worldwide suffer from RCC, yet no FDA-approved therapies exist. Clinical care relies on off-label neuromodulators with modest efficacy, high discontinuation rates, and limited durability.
Meanwhile, late-stage assets are approaching regulatory decision points that could define an entirely new therapeutic class as early as 2026.
The report’s core insight is that RCC is not a signal-to-noise problem. High placebo response, heightened FDA scrutiny following multiple candidate failures, and payer skepticism around durability have compressed the field to what is realistically a one- or two-winner market. In that context, marginal differences in placebo-adjusted efficacy, tolerability, and persistence translate directly into outsized commercial outcomes.
From a market perspective, the numbers are no longer trivial. The RCC treatment market is estimated at ~$9B today across major markets, with projections of $14–15B by 2035, even under conservative assumptions. The report argues that most published forecasts undercount the commercially reachable population, which may be closer to 20–30 million patients globally once diagnostic pathways and referral patterns evolve.
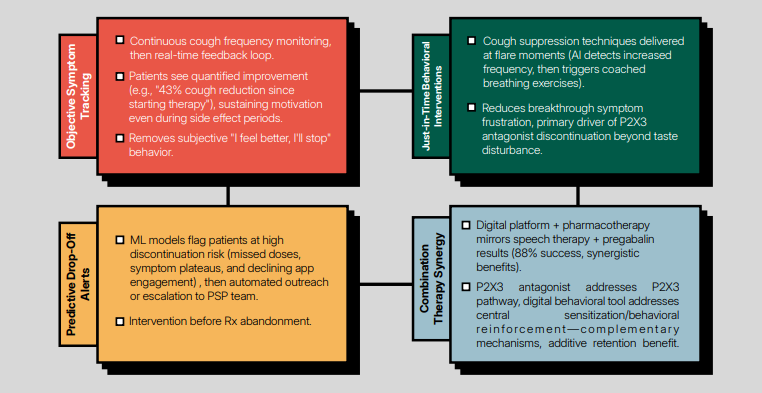
The report also highlights the opportunities around digital therapeutics. Rather than treating digital tools as adjuncts, the analysis positions them as structural infrastructure: expanding access where behavioral therapy capacity is constrained, generating objective real-world evidence, and materially improving treatment persistence. In a category where durability increasingly determines pricing and reimbursement, this is significant.
The takeaway for investors and pharma executives is not that RCC is suddenly low-risk. It isn’t. But the rules are changing. Success will depend less on novelty and more on demonstrating durable, placebo-adjusted efficacy at scale, paired with evidence that payers can trust.
That combination is rare. Which is precisely why chronic cough is finally worth serious attention.
The full report is available free at coughreport.com.
Our latest news
Lorem ipsum dolor sit amet, consectetur adipiscing elit. Non eget pharetra nibh mi, neque, purus.



