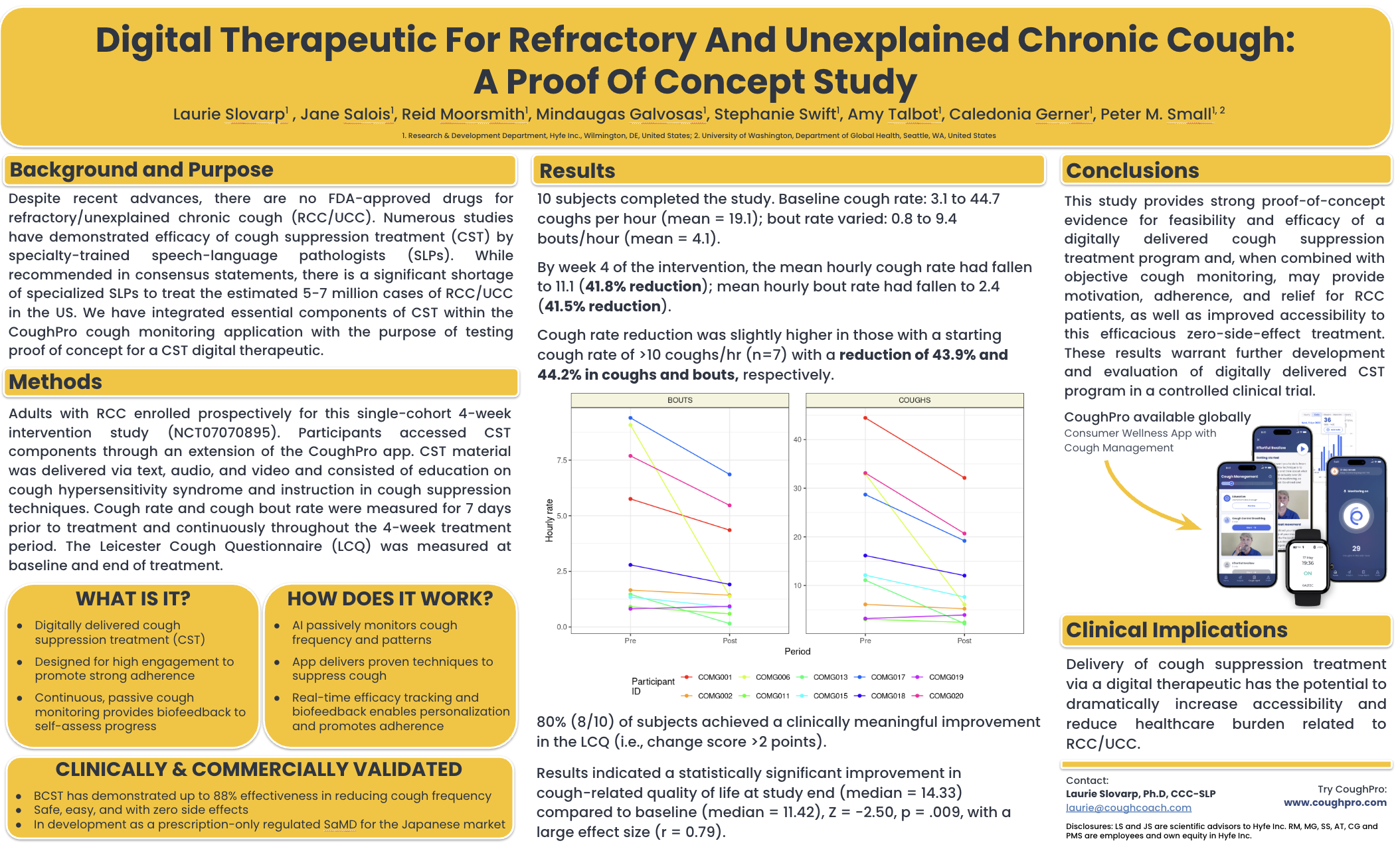
ATS 2025: A Proof of Concept Therapeutic Solution for Chronic Cough: Personalized Monitoring and Targeted Suppression
Summary
The world’s first DTx for Refractory/Unexplained Chronic Cough (RCC/UCC). What it is? Digitally delivered behavioral cough suppression therapy (BCST), designed for high engagement to promote strong adherence. It includes continuous, passive cough monitoring and biofeedback loop enabling tailored personalization.
Unmet needs in chronic cough are significant, affecting millions globally:
- ~7M RCC/UCC patients in the US (~200M globally)
- No approved antitussive drugs for RCC/UCC
- Guideline‑recommended behavioral therapy for RCC/UCC is proven - just largely unavailable (<250 providers in the US)
Behavioral Cough Suppression Therapy (BCST) is Proven To Work
- >15 studies (4 RCTs, 11+ prospective cohorts/series, 4 languages) with ~500 unique patients evaluated
- Mean LCQ improvement: +4 - 7.5 points (3-4x MCID)
- Mean 24-h cough-count reduction: 30-50% (41% in largest RCT)
- No adverse events - excellent safety
Digital Therapeutic Opportunity - why now?
- Proven mechanism – neuroplastic retraining of cough reflex
- Compelling effect size – equal or superior to top antitussive molecules in development, potential for “software + pill”
- No safety liabilities
- Telehealth validation – 88% responder rate in video-BCST cohort
- Pathways to reimbursement are increasing worldwide
WHAT IT IS?
- Digitally delivered behavioral cough suppression therapy (BCST)
- Designed for high engagement to promote strong adherence
- Continuous, passive cough monitoring
- Biofeedback loop enabling tailored personalization
HOW IT WORKS?
- AI passively monitors cough frequency and patterns
- App delivers proven techniques to suppress cough
- Real-time efficacy tracking and biofeedback enables personalization and ensures adherence
CLINICALLY & COMMERCIALLY VALIDATED
- BCST has demonstrated up to 88% effectiveness in reducing cough frequency
- Safe, easy, and with zero side effects
- In development as a prescription-only regulated SaMD for the Japanese market