ATS 2025: ARINA-1 Improves Quality of Life in a Pilot Study of Chronic Bronchitis
Summary
This study used Hyfe's wearable cough monitor during a 7-day run in, 28-day treatment, and 14-day follow-up period in patients with chronic bronchitis.
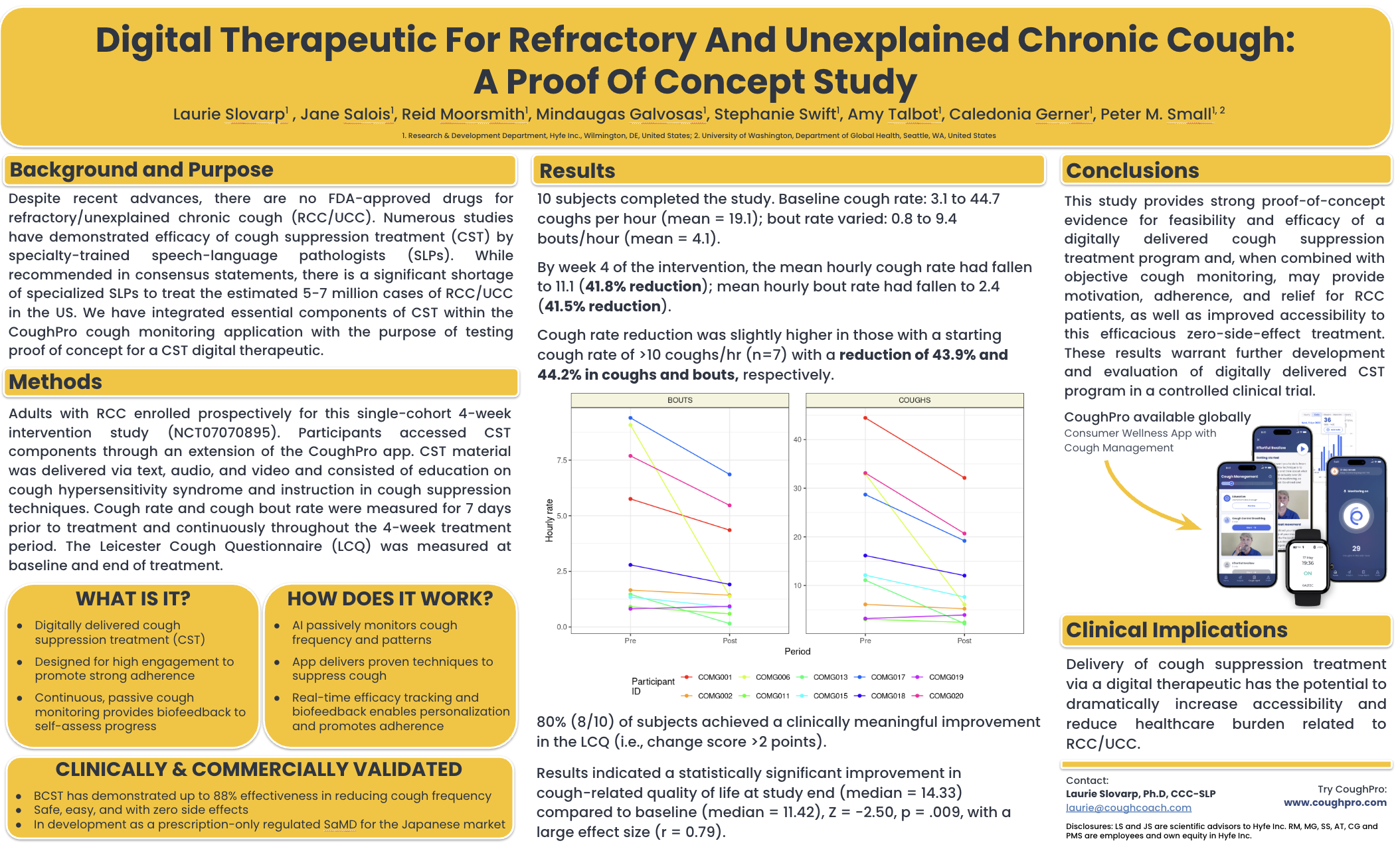
Chronic bronchitis (CB) is defined by a chronic cough lasting > 3 months in a year for 2 successive years without another cause. The pathology of CB involves enlargement and hypertrophy of submucosal mucus glands, increased numbers ofgoblet cells, and increased airways inflammation. Current therapies for the prominent cough are largely ineffective for many patients. ARINA-1 is a buffered fixed dose combination therapy of ascorbic acid and reduced glutathione that is nebulized twice daily via an Investigational PARI eFlow nebulizer. In addition to CB, it is currently being evaluated in non-CFbronchiectasis and lung transplantation. Studies have shown that ARINA-1 improves mucociliary clearance superior tohypertonic saline and downregulates inflammatory signaling. Thus, ARINA-1 may improve quality of life in CB by enabling mucus clearance and decreasing inflammatory signaling. This study was an open-label pilot study of ARINA-1 in six optimally treated adult participants with a primary diagnosis of CB, an FEV1 > 50% predicted, and a summary score of>15 points on the Ease of Cough and Sputum Questionnaire (MCQ). Participants were excluded if they were current smokers or had an active exacerbation. Enrolled participants used ARINA-1 twice daily for 28 days and were followed foran additional 28 days off therapy. A wearable cough monitor (Hyfe, Wilmington, DE) was worn during a 7-day run in, 28-day treatment, and 14-day follow-up period. The MCQ and Chronic Airways Assessment Test (CAAT) were administered at baseline, day 28, and day 56. Safety, including spirometry, was monitored throughout the study. Participants improved theMQC (-5.00 ± 1.1, mean ± SD) and CAAT (-8.67 ± 5.13) at 28 days with all participants improving scores > than the minimal clinically important difference (MCID; -2 points for each questionnaire). On day 56, the MCQ scores returned to baseline (-1.17 ± 2.23). The CAAT scores on day 56 remained in the MCID range (-4.17 ± 2.32). A daily diary showed an improved sensation of mucus clearance from the first dose of ARINA-1 in all participants. There were no changes in lung function, but cough count decreased by >30% in 6/6 subjects. A single participant requested and was given an antibiotic for7 days in week 2. Overall, ARINA-1 demonstrated a strong safety profile in patients with CB and improved quality of life and decreased cough frequency. These data demonstrate the need for further study of the use of ARINA-1 in CB